in Slovakia
are Europe’s top products
Book an appointment
(+421 2 20670 176, 187)
Accommodation
Patient safety
Patient safety is essential for providing high‑quality basic health services.
4 000+
EXAMINATIONS
PER YEAR
52
EXPERTS
ANDSCIENTISTS
7 700+
ANNUAL PRODUCTION
OF RADIOPHARMACEUTICALS
17
YEARS
OF EXPERIENCE
3000+
Vyšetrení ročne
62
odborníkov a vedcov
1200+
ročná produkcia rádiofarmák
17
rokov skúseností

About us.
More information
Technologies for life.

Public accessible expert information servise
Pharmacovigilance
Reporting adverse drug effects
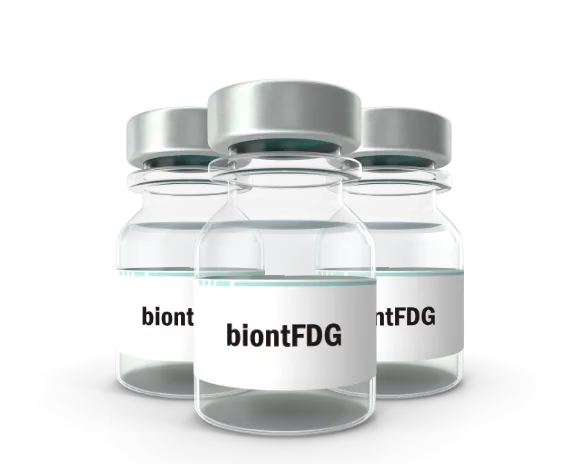
BiontFDG
and cooperation.
BIONT’s own product – biontFDG radiopharmaceutical – is already registered in six EU countries. Since 2012, it has been manufactured under license by the company Eczacibasi-Monrol in Romania and is currently building new factories in Bulgaria and Poland. In addition to the standard PET/CT examination using FDG, BIONT provides examination using 11C-Methionine and expands its production to include other PET radiopharmaceuticals. BIONT is also a contract manufacturer of FDG for Radiomedic (Czech Republic) and DSD Pharma (Austria).
